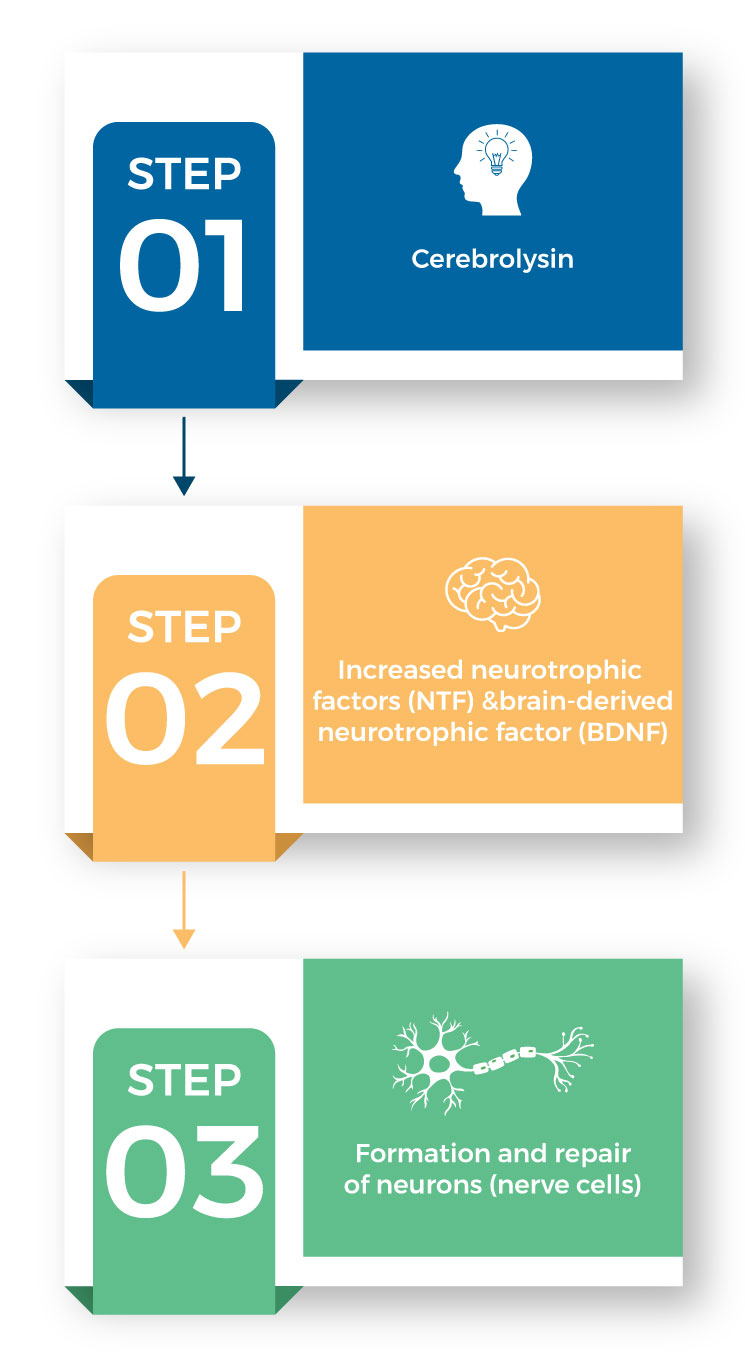
-
Xiao S, Xue H, Li G. Therapeutic effects of cerebrolysin added to risperidone in patients with schizophrenia dominated by negative symptoms. The Australian and New Zealand journal of psychiatry. 2012; 46(2):153-60. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22311531
-
Crook TH, Ferris SH, Alvarez XA, Laredo M, Moessler H. Effects of N-PEP-12 on memory among older adults. International clinical psychopharmacology. 2005; 20(2):97-100. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15729085
-
Alvarez XA, Lombardi VR, Corzo L. Oral Cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. Journal of neural transmission. Supplementum. 2000; 59:315-28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10961443
-
Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia. Drugs of today (Barcelona, Spain: 1998). 2012; 48 Suppl A:25-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22514793
-
Rockenstein E, Torrance M, Mante M. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. Journal of neuroscience research. 2006; 83(7):1252-61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16511867
-
Rockenstein E, Mallory M, Mante M. Effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer’s disease. Journal of neural transmission. Supplementum. 2002. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12456076.
-
Rockenstein E, Adame A, Mante M, Moessler H, Windisch M, Masliah E. The neuroprotective effects of Cerebrolysin in a transgenic model of Alzheimer’s disease are associated with improved behavioral performance. Journal of neural transmission (Vienna, Austria : 1996). 2003; 110(11):1313-27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14628195
-
Ladurner G, Kalvach P, Moessler H, .Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. Journal of neural transmission (Vienna, Austria : 1996). 2005; 112(3):415-28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15583955
-
Muresanu DF, Heiss W-D, Hoemberg V, et al. Cerebrolysin and Recovery After Stroke (CARS): A Randomized, Placebo-Controlled, Double-Blind, Multicenter Trial. Stroke; a Journal of Cerebral Circulation. 2016;47(1):151-159. doi:10.1161/STROKEAHA.115.009416. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689177/.
-
Guekht A, Vester J, Heiss WD. Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2017; 38(10):1761-1769. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28707130.
-
Chen CC, Wei ST, Tsaia SC, Chen XX, Cho DY. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. British journal of neurosurgery. 2013; 27(6):803-7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23656173.
-
Bornstein N, Poon WS. Accelerated recovery from acute brain injuries: clinical efficacy of neurotrophic treatment in stroke and traumatic brain injuries. Drugs of today (Barcelona, Spain : 1998). 2012; 48 Suppl A:43-61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22514794
-
Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Actaneurochirurgica. Supplement. 2005; 95:59-60. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16463821.
-
Zhang D, Dong Y, Li Y, Chen J, Wang J, Hou L. Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials. BioMed Research International. 2017;2017:4191670. doi:10.1155/2017/4191670. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5474547/.
-
Hassanein SM, Deifalla SM, El-Houssinie M, Mokbel SA. Safety and Efficacy of Cerebrolysin in Infants with Communication Defects due to Severe Perinatal Brain Insult: A Randomized Controlled Clinical Trial. Journal of clinical neurology (Seoul, Korea). 2016; 12(1):79-84. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26365023.
-
Ozkizilcik A, Sharma A, Muresanu DF. Timed Release of Cerebrolysin Using Drug-Loaded TitanateNanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28875428.
-
Ozkizilcik A, Sharma A, Muresanu DF. Timed Release of Cerebrolysin Using Drug-Loaded TitanateNanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28875428.
-
Requejo C, Ruiz-Ortega JA, Cepeda H. Nanodelivery of Cerebrolysin and Rearing in Enriched Environment Induce Neuroprotective Effects in a Preclinical Rat Model of Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28840482.
-
Noor NA, Mohammed HS, Mourad IM, Khadrawy YA, AboulEzz HS. A promising therapeutic potential of cerebrolysin in 6-OHDA rat model of Parkinson’s disease. Life sciences. 2016; 155:174-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27210889
-
KalynIaB, Safarova TP, Sheshenin VC, Gavrilova SI. [Comparative efficacy and safety of antidepressant mono- and multimodal therapy in elderly patients with depression (a clinical experience in a psychogeriatric hospital)]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2014; 114(6 Pt 2):20-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25042499.
-
Gharagozli, K., Harandi, A. A., Houshmand, S., Akbari, N., Muresanu, D. F., Vester, J., Winter, S., & Moessler, H. (2017). Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial. Journal of medicine and life, 10(3), 153–160.
-
Masliah, E., & Díez-Tejedor, E. (2012). The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs of today (Barcelona, Spain: 1998), 48 Suppl A, 3–24. https://doi.org/10.1358/dot.2012.48(Suppl.A).1739716
-
Fiani, B., Covarrubias, C., Wong, A., Doan, T., Reardon, T., Nikolaidis, D., & Sarno, E. (2021). Cerebrolysin for stroke, neurodegeneration, and traumatic brain injury: review of the literature and outcomes. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 42(4), 1345–1353. https://doi.org/10.1007/s10072-021-05089-2
-
Zhang, C., Chopp, M., Cui, Y., Wang, L., Zhang, R., Zhang, L., Lu, M., Szalad, A., Doppler, E., Hitzl, M., & Zhang, Z. G. (2010). Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. Journal of neuroscience research, 88(15), 3275-3281. https://doi.org/10.1002/jnr.22495.
-
Bornstein, N. M., Guekht, A., Vester, J., Heiss, W. D., Gusev, E., Hömberg, V., Rahlfs, V. W., Bajenaru, O., Popescu, B. O., & Muresanu, D. (2018). Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 39(4), 629–640. https://doi.org/10.1007/s10072-017-3214-0.
-
Heiss, W. D., Brainin, M., Bornstein, N. M., Tuomilehto, J., Hong, Z., & Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators (2012). Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke, 43(3), 630–636. https://doi.org/10.1161/STROKEAHA.111.628537.
-
Chang, W. H., Lee, J., Shin, Y. I., Ko, M. H., Kim, D. Y., Sohn, M. K., Kim, J., & Kim, Y. H. (2021). Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. Journal of personalized medicine, 11(6), 545. https://doi.org/10.3390/jpm11060545
-
Tran, L., Alvarez, X. A., Le, H. A., Nguyen, D. A., Le, T., Nguyen, N., Nguyen, T., Nguyen, T., Vo, T., Tran, T., Duong, C., Nguyen, H., Nguyen, S., Nguyen, H., Le, T., Nguyen, M., & Nguyen, T. (2022). Clinical Efficacy of Cerebrolysin and Cerebrolysin plus Nootropics in the Treatment of Patients with Acute Ischemic Stroke in Vietnam. CNS & neurological disorders drug targets, 21(7), 621–630. https://doi.org/10.2174/1871527320666210820091655
-
Stan, A., Birle, C., Blesneag, A., & Iancu, M. (2017). Cerebrolysin and early neurorehabilitation in patients with acute ischemic stroke: a prospective, randomized, placebo-controlled clinical study. Journal of medicine and life, 10(4), 216–222.
-
Chen, N., Yang, M., Guo, J., Zhou, M., Zhu, C., & He, L. (2013). Cerebrolysin for vascular dementia. The Cochrane database of systematic reviews, (1), CD008900. https://doi.org/10.1002/14651858.CD008900.pub2.
-
Lang, W., Stadler, C. H., Poljakovic, Z., Fleet, D., & Lyse Study Group (2013). A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy of combined treatment with alteplase (rt-PA) and Cerebrolysin in acute ischaemic hemispheric stroke. International journal of stroke: official journal of the International Stroke Society, 8(2), 95–104. https://doi.org/10.1111/j.1747-4949.2012.00901.x.
-
Panisset M, Gauthier S, Moessler H, Windisch M, .Cerebrolysin in Alzheimer’s disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent. Journal of neural transmission (Vienna, Austria: 1996). 2002; 109(7-8):1089-104. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12111446.
-
Retrieved from http://www.wfsbp.org/doi/wfsbp2011-abstractscd/en/abstracts/10025.html.
-
Shabanov PD, Lebedev AA, Pavlenko VP, Ganapol’skiĭ VP. [Comparative study of behavioral effects of cortexin and cerebrolysine upon intraventricular and intraperitoneal administration in rats]. Eksperimental’naia i klinicheskaiafarmakologiia. ; 70(3):13-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17650626
-
Krasnoperova MG, Bashina VM, Skvortsov IA, Simashkova NV. [The effect of cerebrolysin on cognitive functions in childhood autism and in Asperger syndrome]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2003; 103(6):15-8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12872620.
-
Chutko LS, Yakovenko EA, Surushkina SY, Kryukova EM, Palaieva SV. [The efficacy of cerebrolysin in the treatment of autism spectrum disorders]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2017; 117(9):71-75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29053124.
-
Cuevas-Olguin R, Roychowdhury S, Banerjee A. Cerebrolysin prevents deficits in social behavior, repetitive conduct, and synaptic inhibition in a rat model of autism. Journal of neuroscience research. 2017; 95(12):2456-2468. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28609577.
-
Sotnikova NY, Gromova OA, Novicova EA. Dual effect of cerebrolysin in children with attention deficit syndrome with hyperactivity: neuroprotection and immunomodulation. Russian journal of immunology: RJI : official journal of Russian Society of Immunology. 2002; 7(4):357-64. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12687248
-
Chutko LS, Yakovenko EA, Surushkina SY, Anisimova TI, Kropotov YD. [Clinical and neurophysiological heterogeneity of attention deficit hyperactivity disorder]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova.; 116(10):117-121. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27845323.
-
Nasiri J, Safavifar F. Effect of cerebrolysin on gross motor function of children with cerebral palsy: a clinical trial. ActaneurologicaBelgica. 2017; 117(2):501-505. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28074392.
-
Gershman RN, Vasilenko MA. [Use of cerebrolysin and ATP in treating infantile cerebral paralysis]. Pediatriiaakusherstvo i ginekologiia.. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1228606.
-
Retrieved from https://cerebralpalsynewstoday.com/2017/06/07/cerebrolysin-can-help-improve-motor-skills-in-cerebral-palsy-patients/.
-
Retrieved from https://clinicaltrials.gov/ct2/show/NCT02116348
-
Biesenbach G, Grafinger P, Eichbauer-Sturm G, Zazgornik J. [Cerebrolysin in treatment of painful diabetic neuropathy]. Wiener medizinischeWochenschrift (1946). 1997; 147(3):63-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9173675.
-
Dong H, Jiang X, Niu C, Du L, Feng J, Jia F. Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy. Neural Regeneration Research. 2016;11(1):156-162. doi:10.4103/1673-5374.175063. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4774211/
-
Retrieved from https://www.researchgate.net/publication/286293430_Cerebrolysin_as_a_nerve_growth_factor_for_treatment_of_acquired_peripheral_nervous_system_diseases.
-
Masliah E, Armasolo F, Veinbergs I, Mallory M, Samuel W. Cerebrolysin ameliorates performance deficits, and neuronal damage in apolipoprotein E-deficient mice. Pharmacology, biochemistry, and behavior. 1999; 62(2):239-45. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9972690.
-
Keilhoff G, Lucas B, Pinkernelle J, Steiner M, Fansa H. Effects of cerebrolysin on motor-neuron-like NSC-34 cells. Experimental cell research. 2014; 327(2):234-55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24997385.
-
Shchudlo NA, Shchudlo MM, Borisova IV. [The effect of cerebrolysin on the regeneration of the peripheral nerve depending on the scheme of paraneural administration]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2013; 113(12):76-80. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24430040.
-
Available from https://journals.lww.com/nrronline/Abstract/2011/06180/Cerebrolysin_as_a_nerve_growth_factor_for.10.aspx.
-
Sharma HS, Sharma A, Mössler H, Muresanu DF. Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: modulatory roles of co-morbidity factors and nanoparticle intoxication. International review of neurobiology. 2012; 102:249-76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22748833.
-
Sharma A, Muresanu DF, Mössler H, Sharma HS. Superior neuroprotective effects of cerebrolysin in nanoparticle-induced exacerbation of hyperthermia-induced brain pathology. CNS & neurological disorders drug targets. 2012; 11(1):7-25. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22229316.
-
Martínez-Laorden E, Hurle MA, Milanés MV, Laorden ML, Almela P. Morphine withdrawal activates hypothalamic-pituitary-adrenal axis and heat shock protein 27 in the left ventricle: the role of extracellular signal-regulated kinase. The Journal of pharmacology and experimental therapeutics. 2012; 342(3):665-75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22647273.
-
Sharma HS, Ali SF, Patnaik R, Zimmermann-Meinzingen S, Sharma A, Muresanu DF. Cerebrolysin Attenuates Heat Shock Protein (HSP 72 KD) Expression in the Rat Spinal Cord Following Morphine Dependence and Withdrawal: Possible New Therapy for Pain Management. Current neuropharmacology. 2011; 9(1):223-35. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21886595.
-
Belokrylov GA, Malchanova IV. [Levamin and cerebrolysin as immunostimulants]. Biulleten’ eksperimental’noibiologii i meditsiny. 1992; 113(2):165-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1611065.
-
Govorin NV, Zlova TP, Akhmetova VV, Tarasova OA. [The pathophysiological analysis of cerebrolysin therapy of children with mental developmental delay caused by ecological factors]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2008; 108(5):51-5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18577958.
-
Sotnikova NY, Gromova OA, Novikova EA, Burtsev EM. Immunoactive Properties of Cerebrolysin. Russian journal of immunology: RJI : official journal of Russian Society of Immunology. 2000; 5(1):63-70. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12687163.
-
Garmanchuk LV, Perepelitsyna EM, SidorenkoMv, Makarenko AN, Kul’chikov AE. [Cytoprotective effect of neuropeptides on immunocompetent cells (in vitro study)]. Eksperimental’naia i klinicheskaiafarmakologiia.; 72(4):28-32. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19803367
-
González ME, Francis L, Castellano O. Antioxidant systemic effect of short-term Cerebrolysin administration. Journal of neural transmission. Supplementum. 1998; 53:333-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9700669.
-
Gromova OA, Avdeenko TV, Burtsev EM, Skal’nyĭ AV, Solov’ev OI. [Effects of cerebrolysin on the oxidant homeostasis, the content of microelements and electrolytes in children with minimal brain dysfunction]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 1998; 98(1):27-30. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9505400.
-
Retrieved from https://www.researchgate.net/publication/270571093_Antioxidant_properties_of_cerebrolysin_-_an_old_drug_with_a_newly_discovered_capabilities
-
González ME, Francis L, Castellano O. Antioxidant systemic effect of short-term Cerebrolysin administration. Journal of neural transmission. Supplementum. 1998; 53:333-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9700669.
-
Retrieved from http://europepmc.org/abstract/med/9505400.
-
Huang TL, Huang SP, Chang CH, Lin KH, Sheu MM, Tsai RK. Protective effects of cerebrolysin in a rat model of optic nerve crush. The Kaohsiung journal of medical sciences. 2014; 30(7):331-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24924838.
-
Retrieved from http://www.roneurosurgery.eu/atdoc/16CostinDCombined.pdf.
 The Denim Shop
The Denim Shop
 Japanese Beauty
Japanese Beauty
 European Beauty
European Beauty
 American Beauty
American Beauty
 Makeup
Makeup
 Fragrances
Fragrances
 Bath & Body
Bath & Body
 Hair Care
Hair Care
 Vitamins & Supplements
Vitamins & Supplements
 Home
Home
 Clearance Sale
Clearance Sale